
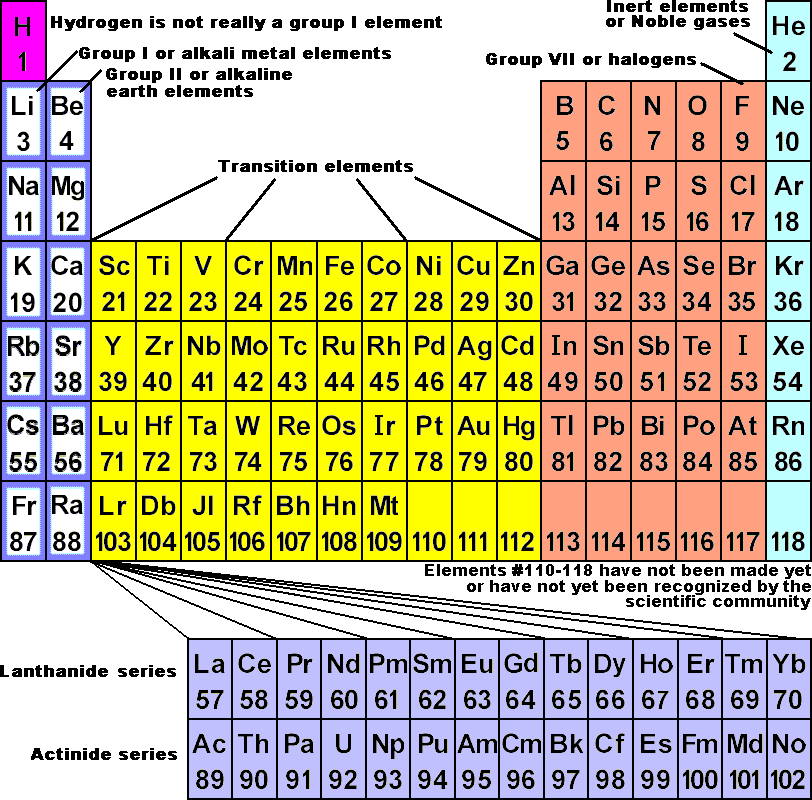
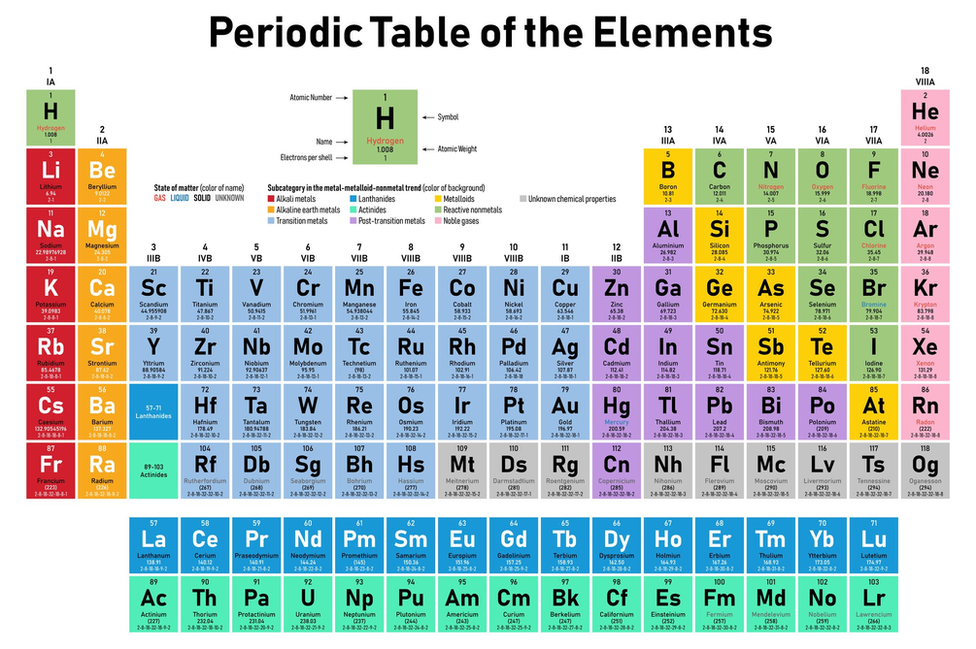
Mendeleev Periodic Table Characteristicsġ. Each column will be subdivided into two sub-columns (A and B), with components from the same sub-columns sharing the most similarities. If the pieces are similar but not identical, they are placed below but somewhat apart to differentiate them. Mendeleev’s periodic table is composed of horizontal rows and vertical columns, or periods and groups.Ĥ. A ‘column’ is constructed by stacking the periods one on top of the other (group). When an element has a high degree of similarity to an earlier element, the new element with comparable qualities is put exactly beneath the earlier element to begin a new period.ģ. A “period” is a horizontal grouping of items.Ģ. The elements are presented in ascending order of atomic mass in a horizontal line.ġ. Reversing the order of the elements in Mendeleev’s periodic table aids comprehension. Mendeleev’s first periodic table, published in 1869, is shown below. Elements with equal properties can be found in the same horizontal row. Elements with similar properties are grouped together. Mendeleev originally arranged elements in ascending order of atomic weight, one beneath the other. Mendeleev’s law states that “the Physical and Chemical Properties of the Elements are Periodic Functions of Their Atomic Weights.” What was Mendeleev’s Elements Arranging Method? What exactly is Mendeleev’s Periodic Law? The element displaying the repeat of the properties was placed in the first slot of this new template. As soon as the qualities of the components were discovered to be duplicated, he stopped that row and began a new one below it. Mendeleev devised a row of elements arranged in ascending atomic weight order. He arranged the elements in the periodic table so that those with similar qualities were grouped together in the same vertical columns. After studying the properties of elements, Mendeleev determined that they were related to atomic mass in a periodic pattern. At the time of Mendeleev, only 63 elements were known. Elements were organised in Mendeleev’s periodic table based on their fundamental properties, atomic mass, and chemical properties. There have been several periodic tables developed, but the Mendeleev periodic table is the most important.įollowing the rejection of Newlands Octave Law in 1869, the Mendeleev Periodic Table was developed. Elements with similar chemical properties appear at regular intervals, within the vertical columns called groups.Mendeleev Periodic Table:- Dmitri Ivanovich Mendeleev, a Russian scientist, was the most essential contributor to the early development of the periodic table. Each new horizontal row of the periodic table corresponds to the beginning of a new period because a new principal energy level is being filled with electrons. The result is the periodic table as we know it today. Mendeleev and Moseley are credited with being most responsible for the modern periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties. So even though tellurium does indeed have a greater atomic mass than iodine, it is properly placed before iodine in the periodic table. Tellurium has an atomic number of 52, while iodine has an atomic number of 53. When ordered by atomic number, the discrepancies within Mendeleev’s table disappeared. He then realized that the elements of the periodic table should be arranged in order of increasing atomic number rather than increasing atomic mass. His results led to the definition of atomic number as the number of protons contained in the nucleus of each atom. Moseley found that there was a relationship between wavelength and atomic number. He would shoot X-rays through crystals of the element and study the wavelengths of the radiation he detected. Just two years later, in 1913, English physicist Henry Moseley (1887-1915) examined x-ray spectra of a number of chemical elements. It was not until 1911 that Rutherford conducted his gold foil experiment that demonstrated the presence of the nucleus in the atom. When Mendeleev put his periodic table together, nobody knew about the existence of the nucleus.
Individual pieces of information takes on broader significance when put together with other parts of the puzzle. As the game progresses, clues are obtained by each player and they then must assemble these clues into a guess as to the criminal. We have all enjoyed playing the game “Clue.” The object of the game is to obtain information about a murder-who did it, where they did it, and what was used as the murder weapon. How are these items related to one another?


 0 kommentar(er)
0 kommentar(er)
